The Recipe for Steel: Chemical Composition & Performance
Introduction
Have you ever asked yourself what really makes a high performance steel pipe different from a normal one? The difference is not in its size or finish, but in the core of its atomic structure. The process of making quality steel is not unlike that of a master chef preparing a signature dish. The quality, flavor and performance of the end product is wholly dependent on the exact proportion of the components.
In this analogy, pure iron (Fe) is the flour for the cake or the stock for the soup. But by itself, it’s fairly soft and nondescript. The trick is to add a tightly controlled recipe of other chemical elements. Some serve as powerful flavor enhancers, others are contaminants that can spoil the entire meal. Knowing this chemical “recipe” is what allows you to understand why a certain steel pipe is strong and ductile, while another might be brittle and fail. So, here are the key “ingredients” that determine how well your steel pipe will perform.
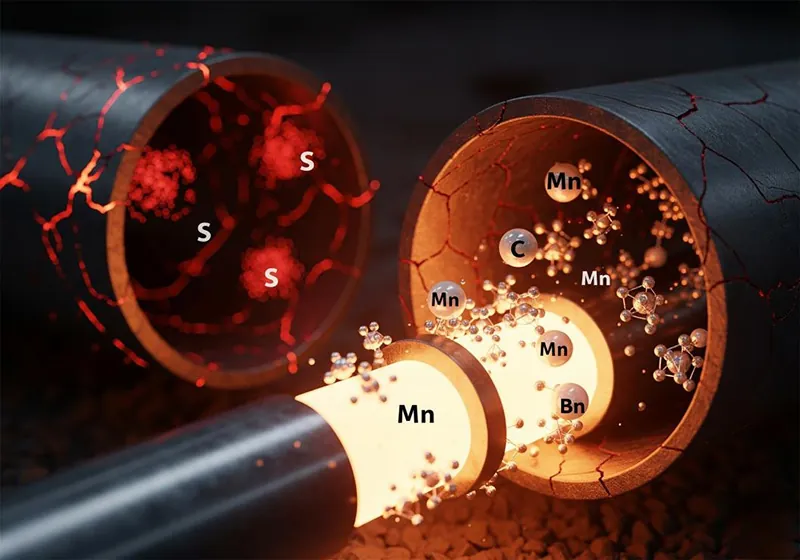
Carbon (C): The Primary Source of Strength
If there’s one thing that defines the nature of steel, it is carbon. Think of it as the salt in the recipe; a little salt goes a long way, and the right amount is everything.
Carbon is the main hardening agent in steel. As you raise the carbon content, you are directly and dramatically raising the strength and hardness of the steel. A higher carbon content means a pipe that can handle higher pressures and more strain, which sounds perfect. But this power has a price.
Just like how too much salt can make a dish bitter and incomestible, too much carbon has some pretty significant downsides. Increased carbon content reduces the ductility of steel, so it becomes more brittle and less able to deform under stress without breaking. More importantly for pipeline building, it drastically reduces the steel’s weldability. High-carbon steels need special, usually expensive, pre-heating and post-welding treatments to avoid the welds to become brittle and to crack. For a job that requires thousands of field welds, that’s a big engineering and financial consideration too. So the carbon content of a pipeline’s steel is a lesson in trade-offs — a delicate balancing act between the strength that’s needed and the toughness and weldability that’s needed.
Manganese (Mn): The Indispensable Helper
If carbon is the main ingredient, manganese is the essential sous-chef that toils behind the scenes. This makes it important and complex when it comes to high quality steelmaking.
For one, manganese is a hardening agent and adds to the overall strength of the steel, similar to carbon, albeit not as much. This enables steelmakers to get the strength they need without pushing the carbon content to levels that would make the steel too brittle.
Second, and maybe more importantly, manganese is a critical “scavenger.” One of the most damaging impurities in steel-making is sulfur (which we’ll get to next). Manganese and Sulfur have a strong attraction and they combine to form manganese sulfide. This is much less detrimental to the properties of steel than the iron sulfide that may have formed. In effect, manganese “neutralizes” the most harmful effects of sulfur, significantly enhancing the quality of the steel and eliminating a form of brittleness that can take place at elevated temperatures (“hot shortness”). It is a critical ingredient in making strong, dependable, and ductile steel.
Sulfur (S) and Phosphorus (P): The Unwanted Impurities
In our cooking metaphor, sulfur and phosphorus are the contaminants you never want in your kitchen—like sand in your flour or grit in your broth. Although they occur naturally in the raw materials (iron ore and coke) used to produce steel, they must be kept to a minimum by extensive refining.
· Sulfur (S): The Enemy of Weldability and Toughness As mentioned, sulfur is a highly undesirable element. The iron sulfides it forms can create weak spots within the steel’s grain structure. These spots can melt at lower temperatures than the steel itself, leading to cracking and defects during the hot rolling or welding process. Even in small amounts, sulfur significantly reduces the steel’s impact toughness, particularly its ability to resist tearing. For any application where reliability is key, the sulfur content must be kept to an absolute minimum, often measured in fractions of a percent.
· Phosphorus (P): The Source of Brittleness Like sulfur, phosphorus is another impurity that must be strictly controlled. While it can slightly increase the strength of steel, this benefit is vastly outweighed by a significant negative: it makes the steel brittle, especially at cold temperatures. This phenomenon, known as “cold shortness,” can make a pipeline dangerously susceptible to fracture in colder climates or when transporting refrigerated products. In virtually all high-quality pipe specifications, the phosphorus content is strictly limited to a very low maximum threshold.
The Balancing Act: Why Steel Grade is All About the Recipe
This leads us to the most important idea: the performance of a steel pipe is not reliant on any one factor, but on the exact, harmonious equilibrium of the entire chemical formula.
This is what a “steel grade” really means. When you order a grade such as API 5L X52 or EN 10219 – S355, you are not simply specifying the mechanical properties; you are requesting a particular, tried and tested chemical composition.The standard specifies the exact permissible range for each significant element – a maximum for impurities such as sulfur and phosphorus and a tightly controlled window for elements such as carbon and manganese.
This certified recipe is what guarantees the final product will have the desired balance of:
· Strength: To handle the operational pressure.
· Toughness/Ductility: To resist fracture and provide a safe failure mode.
· Weldability: To allow for efficient and reliable construction in the field.
The chemical composition is the foundational DNA of the steel. It is the invisible blueprint that dictates the visible mechanical properties and, ultimately, the safety and longevity of your project.
(Conclusion)
Just as a master chef’s recipe is a fine balance of potent ingredients and undesirable impurities, the chemical composition of a steel pipe is a delicate equilibrium of strong chemicals and harmful substances. Carbon is the main source of strength, manganese provides toughness and cleans up contaminants, and sulfur and phosphorus are reduced to almost zero levels to prevent brittleness and defects.
Next time you open up a Mill Test Certificate (MTC) and see the column of chemical analysis data, you will appreciate its profound importance. Those are not trivialities at the tenths of a percent level. They are the fundamental promise of your pipe’s performance, the formula for its strength, and the cornerstone of the integrity of your project.
Get Your Custom Steel Pipe Quote Today!
Provide us with your project details (like application, specifications, quantity). Our experienced team will respond with a tailored solution and competitive quote within 24 business hours.
Related Articles
ASTM A53 vs. API 5L: A Guide to Selection and Application
Introduction:Technology differences determine success or failure, and selection needs to be “precise”
Steel Density Analysis: Core Differences between Mild and Medium Carbon Steels and Industrial Applications
3LPE coated steel pipe: a solid barrier in the field of industrial corrosion protection
3LPP coated pipe: anti-corrosion guard in high temperature and high pressure environment
FBE steel pipe: the technological armor of the steel defense line
HOT TAGS
latest posts
- 3LPP coated pipe: anti-corrosion guard in high temperature and high pressure environment
- ASTM A53 LSAW Steel Pipe Selection Guide for Oil and Gas Transportation Pipelines
- API 5L LSAW Pipe: A Deep Dive into PSL1 vs. PSL2
- Steel Pipe Sizing Errors? DN vs. OD Explained for Buyers
- NDT for Pipe Welds: X-ray vs. Ultrasonic Testing